Illustrative Image: Effect of Radiation and Chemical Reactions on Ozone Layer Healing: Insights from Mathematical Modeling and Climate Policy
Image Source & Credit: MIT
Ownership and Usage Policy
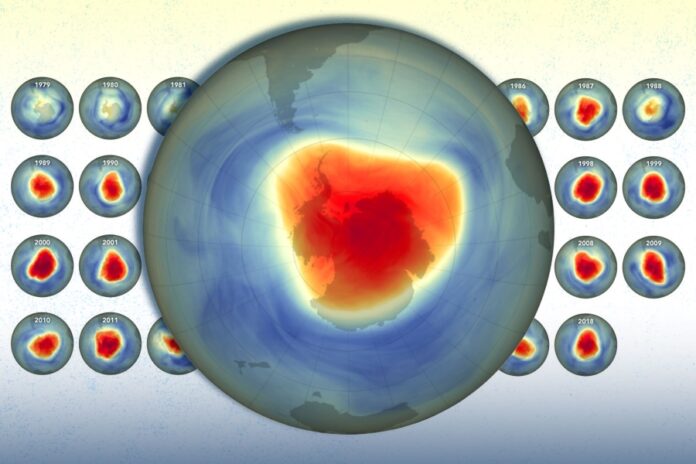
A recent study by Ojo, A. S., & Egbo, C. A. (2025) titled “EFFECT OF RADIATION AND CHEMICAL REACTION ON OZONE LAYER HEALING” published in Open Journal of Physical Science (ISSN: 2734-2123), reveals that radiation accelerates ozone healing, while chemical reactions can either hinder or support recovery, depending on conditions.
“
Radiation accelerates ozone healing, while chemical reactions either hinder or support recovery, highlighting pollution reduction’s importance for atmospheric restoration.
– Ojo, A. S., & Egbo, C. A. (2025
The study examines how environmental factors—particularly radiation and chemical interactions—affect the recovery of the ozone layer. Its primary goal is to understand how solar or cosmic radiation influences ozone restoration and how atmospheric chemical reactions, especially those involving ozone-depleting substances such as chlorofluorocarbons (CFCs) and nitrogen oxides, either accelerate or hinder this healing process. The research focuses on the interaction between radiation and atmospheric molecules, analyzing how incoming energy drives ozone formation or depletion, alongside the complex chemical dynamics that govern these processes. It also explores the natural and human-driven mechanisms that contribute to ozone recovery. By uncovering these dynamics, the study provides valuable insights for environmental protection and climate policy. Its findings could strengthen global initiatives such as the Montreal Protocol, improve strategies for reducing harmful pollutants, and enhance predictions about future ozone recovery trends—crucial for safeguarding life on Earth against harmful ultraviolet radiation.
How the Study was Conducted
The authors employed a mathematical modeling approach to investigate ozone layer healing. They formulated differential equations to describe the variation of ozone concentration over time, incorporating radiation intensity and chemical reaction rates as key variables. By adjusting parameters such as reaction rate constants, radiation absorption coefficients, and initial ozone levels, they conducted a parameter analysis to evaluate their influence on ozone recovery. The equations were then solved using numerical simulation techniques, generating data that illustrated how ozone concentration evolved under different conditions. These outcomes were interpreted graphically, with plots showing ozone variation over time across multiple scenarios. Finally, a comparative evaluation was carried out, contrasting cases with and without radiation and chemical reactions to demonstrate their individual and combined impacts on the healing process.
What the Authors Found
The study revealed several critical insights into the dynamics of ozone recovery. Radiation, particularly from the sun, was shown to accelerate healing by energizing oxygen atoms, enabling them to recombine into ozone molecules. Chemical reactions, however, produced mixed outcomes: while pollutants such as chlorofluorocarbons (CFCs) and nitrogen oxides hindered recovery by breaking down ozone, natural reactions in cleaner atmospheric conditions supported its formation. The research further highlighted the importance of the combined effects of radiation and chemistry, emphasizing that reducing harmful emissions while maintaining natural radiation exposure is key to optimizing ozone restoration. Importantly, the mathematical models employed were able to replicate real-world ozone behavior, validating both the methodology and the study’s conclusions.
Why is this important
Protects Human Health: A healthy ozone layer reduces UV exposure, which helps prevent skin cancer, cataracts, and immune system suppression.
Safeguards Ecosystems: UV radiation can damage crops, marine life (especially plankton), and disrupt food chains. Healing the ozone layer helps preserve biodiversity.
Informs Climate Policy: By showing how radiation and chemical reactions affect ozone dynamics, the study supports smarter environmental regulations and global agreements like the Montreal Protocol.
Predicts Future Trends: The mathematical models used can forecast how quickly the ozone layer might recover under different scenarios, helping scientists and policymakers plan ahead.
Highlights Human Impact: It underscores how pollutants like CFCs and nitrogen oxides can delay healing, reinforcing the need for continued vigilance in reducing emissions.
What the Authors Recommended
- The authors emphasize the importance of minimizing the release of harmful chemicals like chlorofluorocarbons (CFCs), halons, and nitrogen oxides that disrupt ozone formation.
- The study encouraging the use of eco-friendly technologies and renewable energy sources can reduce the chemical pollutants that interfere with ozone healing.
- The study reinforces the value of international protocols especially the Montreal Protocol as effective tools for regulating and phasing out ozone-depleting substances.
- Continued scientific investigation into the interactions between radiation and atmospheric chemistry is recommended to refine predictive models and improve policy decisions.
- Educating communities about the importance of the ozone layer and how everyday actions affect it can foster more responsible environmental behavior.
In conclusion, the study by Ojo and Egbo (2025) underscores the delicate balance between natural processes and human activity in shaping the future of the ozone layer. By demonstrating how radiation accelerates healing while harmful chemical reactions delay it, the research highlights both the progress achieved through global agreements like the Montreal Protocol and the urgent need for continued vigilance in reducing pollutant emissions. Through mathematical modeling and scientific insight, this work not only advances understanding of ozone dynamics but also provides a roadmap for policymakers, scientists, and communities to safeguard the planet from harmful ultraviolet radiation and ensure a healthier environment for generations to come.















 The African Research (AR) Index is a comprehensive scholarly directory and database focused explicitly on journal publishers that publish and disseminate African research.
The African Research (AR) Index is a comprehensive scholarly directory and database focused explicitly on journal publishers that publish and disseminate African research.

