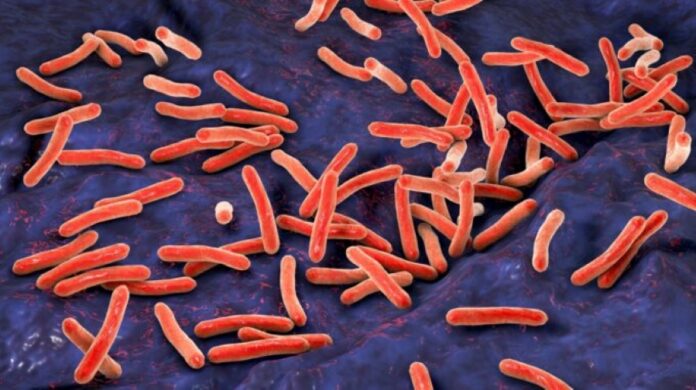
A recent article by Palekyte, et al., (2024) titled “Acetic Acid Enables Molecular Enumeration of Mycobacterium Tuberculosis from Sputum and Eliminates the Need for a Biosafety Level 3 Laboratory” published in Clinical Chemistry, examines that acetic acid is effective in killing Mycobacterium tuberculosis in sputum and preserving RNA for analysis, enabling processing at a lower biosafety level (BSL-2).
“
READ-TB assay uses acetic acid to safely process TB samples at BSL-2, enhancing accessibility.– Palekyte, et al., 2024
The article highlights a significant advancement in tuberculosis (TB) treatment monitoring with the development of the READ-TB assay. This innovative study introduces the use of acetic acid to enumerate Mycobacterium tuberculosis from sputum samples, enabling sample processing at a lower biosafety level (BSL-2) instead of the more stringent BSL-3. The READ-TB assay is semi-automated, rapid, and demonstrates strong correlation with the previously established molecular bacterial load assay (MBLA). This makes it a more accessible and practical option for TB treatment monitoring, particularly in low- and middle-income countries.
One of the key breakthroughs of this assay is the dual role of acetic acid, which not only effectively kills the TB bacteria but also preserves mycobacterial RNA. This preservation ensures accurate enumeration of bacteria even after long-term storage at various temperatures, enhancing the reliability of the results. The READ-TB assay’s ability to function effectively without the need for BSL-3 laboratories is poised to transform TB treatment monitoring in resource-limited settings.
Moreover, the introduction of this assay is expected to enhance the efficiency of clinical trials for new anti-TB drugs by providing a reliable and accessible method for monitoring treatment progress. With its potential to improve TB treatment outcomes and streamline clinical research, the READ-TB assay represents a significant leap forward in the global fight against tuberculosis.
How the Study was Conducted
Human sputum samples were obtained, anonymized, and authorized for assay development. They were spiked with H37Rv or BCG and preserved using various methods for RNA extraction. The authors tested acetic acid as a bactericidal agent that preserves mycobacterial RNA. It was compared to guanidine thiocyanate (GTC) and DNA/RNA Shield for its effectiveness. The Rapid Enumeration and Diagnostic for Tuberculosis (READ-TB) assay was developed. It is semiautomated, rapid, and correlates with the Molecular Bacterial Load Assay (MBLA) when tested on human sputum samples. The authors validated READ-TB and compared it directly to MBLA using sputum collected from individuals with tuberculosis5. The study also tested compatibility with the GeneXpert MTB/RIF Ultra test
What the Authors Found
The authors of the study found that acetic acid is effective in killing Mycobacterium tuberculosis in sputum and preserving RNA for analysis, enabling processing at a lower biosafety level (BSL-2). The study posit that the Rapid Enumeration and Diagnostic for Tuberculosis (READ-TB) assay is a semiautomated, rapid method that correlates well with the Molecular Bacterial Load Assay (MBLA) for monitoring tuberculosis treatment. In addition, RNA from sputum samples treated with acetic acid remains stable for 2 weeks at 25°C and for a year at -20°C, which is beneficial for laboratories without access to -70°C freezers3. Furthermore, READ-TB can be adopted by clinical microbiology laboratories, especially in low- and middle-income countries, to monitor tuberculosis treatment without the need for a BSL-3 lab4. It is also compatible with the GeneXpert MTB/RIF Ultra test5.
Why is this Important
Improved TB Treatment Monitoring: The development of the READ-TB assay allows for more efficient and accessible monitoring of tuberculosis treatment. By using acetic acid to preserve mycobacterial RNA, laboratories can process samples at a lower biosafety level (BSL-2) instead of requiring a BSL-3 lab. This is especially significant for settings with limited resources or lacking BSL-3 facilities.
Global Impact: Tuberculosis remains a major global health concern, affecting millions of people each year. The READ-TB assay’s compatibility with the GeneXpert MTB/RIF Ultra test and its potential adoption by clinical microbiology laboratories in low- and middle-income countries could significantly enhance TB management and patient outcomes.
Scientific Advancement: The study demonstrates innovative approaches to TB diagnosis and monitoring. The use of acetic acid as a bactericidal agent and RNA preservative opens up new possibilities for other infectious disease assays and research.
What the Authors Recommend
The article doesn’t explicitly mention recommendations made by the authors. However, based on the study’s findings, here are some potential recommendations:
- The authors might recommend the adoption of the READ-TB assay in clinical microbiology laboratories, especially in resource-constrained settings. This would allow for more accessible and efficient tuberculosis treatment monitoring.
- Given the promising results, the authors may suggest further research to validate the assay’s performance across diverse populations, including different TB strains and patient demographics.
- Collaboration with public health agencies, researchers, and policymakers could help implement the READ-TB assay in real-world scenarios and improve TB management.
In conclusion, the study by Palekyte et al. represents a significant leap forward in tuberculosis treatment and monitoring. The innovative READ-TB assay, utilizing acetic acid to preserve mycobacterial RNA, enables safe and efficient processing of sputum samples at a lower biosafety level. This advancement holds great promise for improving TB management, particularly in resource-constrained settings, and enhancing the efficacy of clinical trials for new treatments. By making advanced TB diagnostics more accessible, the READ-TB assay has the potential to transform global TB care and significantly impact public health outcomes.















 The African Research (AR) Index is a comprehensive scholarly directory and database focused explicitly on journal publishers that publish and disseminate African research.
The African Research (AR) Index is a comprehensive scholarly directory and database focused explicitly on journal publishers that publish and disseminate African research.

